Tetrahedral: four bonds on one central atom with bond angles of 109.5°. Trigonal bipyramidal: five atoms around the central atom; three in a plane with bond angles of 120° and two on opposite ends of the molecule. Octahedral: six atoms around the central atom, all with bond angles of 90°. This is the AtomCentral trailer. We made the cult documentary 'Trinity and Beyond' and 4 other documentaries on the nuclear era as well as provide research for television and film and the. The way I like to determine hybridization is through something known as the “steric number”, or the number of “things” around a a central atom. Steric number = number of bonded atoms + number of lone pairs. For example, in SiCl4, there are four at.
CHEMISTRY THE CENTRAL SCIENCE
8 BASIC CONCEPTS OF CHEMICAL BONDING
EXERCISES
VISUALIZING CONCEPTS
8.1 For each of these Lewis symbols, indicate the group in the periodic table in which the element X belongs: [Section 8.1]
8.2 Illustrated are four ions—A, B, X, and Y—showing their relative ionic radii. The ions shown in red carry positive charges: a 2+ charge for A and a 1+ charge for B. Ions shown in blue carry negative charges: a 1- charge for X and a 2- charge for Y. (a) Which combinations of these ions produce ionic compounds where there is a 1:1 ratio of cations and anions? (b) Among the combinations in part (a), which leads to the ionic compound having the largest lattice energy? (c) Which combination of ions leads to the ionic compound having the smallest lattice energy? [Section 8.2]
8.3 A portion of a two-dimensional “slab” of NaCl(s) is shown here (see Figure 8.3) in which the ions are numbered. (a) Of the following types of interactions (identified by color), which are attractive and which are repulsive: “purple-purple,” “purple-green,” “green-green”? Explain.(b) Consider the “green-green” interactions between ions 1 and 3, ions 1 and 5, and ions 3 and 5. Which one or more of these three will result in the interaction of largest magnitude? Which one or more will result in the interaction of the smallest magnitude? (c) Consider the “green-green” interactions between ions 1 and 5 and the “green-purple” interactions between ions 1 and 2. Which of these will have the greater magnitude? (d) Does your answer to part (c) help explain why NaCl is a stable ionic solid? [Section 8.2]
8.4 The orbital diagram that follows shows the valence electrons for a 2+ ion of an element. (a) What is the element? (b) What is the electron configuration of an atom of this element? [Section 8.2]
8.5 In the Lewis structure shown here, A, D, E, Q, X, and Z represent elements in the first two rows of the periodic table (H− Ne). Identify all six elements so that the formal charges of all atoms are zero. [Section 8.3]
8.6 Incomplete Lewis structures for the nitrous acid molecule, HNO2, and the nitrite ion, , are shown below. (a) Complete each Lewis structure by adding electron pairs as needed. (b) Is the formal charge on N the same or different in these two species? (c) Would either HNO2 or be expected to exhibit resonance? (d) Would you expect the N=O bond in HNO2 to be longer, shorter, or the same length as the N—O bonds in ? Explain. [Sections 8.5 and 8.6]
N—O—N=O O—N=O
8.7 The partial Lewis structure that follows is for a hydrocarbon molecule. In the full Lewis structure, each carbon atom satisfies the octet rule, and there are no unshared electron pairs in the molecule. The carbon–carbon bonds are labeled 1, 2, and 3. (a) Determine where the hydrogen atoms are in the molecule. (b) Rank the carbon–carbon bonds in order of increasing bond length. (c) Rank the carbon–carbon bonds in order of increasing bond enthalpy. [Sections 8.3 and 8.8]
8.8 Consider the Lewis structure for the polyatomic oxyanion shown here, where X is an element from the third period (Na—Ar). By changing the overall charge, n, from 1– to 2– to 3– we get three different polyatomic ions. For each of these ions (a) identify the central atom, X; (b)determine the formal charge of the central atom, X; (c) draw a Lewis structure that makes the formal charge on the central atom equal to zero. [Sections 8.5, 8.6, and 8.7]
LEWIS SYMBOLS (section 8.1)
8.9(a) What are valence electrons? (b) How many valence electrons does a nitrogen atom possess? (c) An atom has the electron configuration 1s22s22p63s23p2. How many valence electrons does the atom have?
8.10 (a) What is the octet rule? (b) How many electrons must a sulfur atom gain to achieve an octet in its valence shell? (c) If an atom has the electron configuration 1s22s22p3, how many electrons must it gain to achieve an octet?
______
8.11 Write the electron configuration for silicon. Identify the valence electrons in this configuration and the nonvalence electrons. From the standpoint of chemical reactivity, what is the important difference between them?
8.12 (a) Write the electron configuration for the element titanium, Ti. How many valence electrons does this atom possess? (b) Hafnium, Hf, is also found in group 4B. Write the electron configuration for Hf. (c) Ti and Hf behave as though they possess the same number of valence electrons. Which of the sub-shells in the electron configuration of Hf behave as valence orbitals? Which behave as core orbitals?
______
8.13 Write the Lewis symbol for atoms of each of the following elements: (a) Al, (b) Br, (c) Ar, (d) Sr.
8.14 What is the Lewis symbol for each of the following atoms or ions: (a) K, (b) As, (c) Sn2+, (d) N3−?
______
IONIC BONDING (section 8.2)
8.15 Using Lewis symbols, diagram the reaction between magnesium and oxygen atoms to give the ionic substance MgO.
8.16 Use Lewis symbols to represent the reaction that occurs between Ca and F atoms.
______
8.17 Predict the chemical formula of the ionic compound formed between the following pairs of elements: (a) Al and F, (b) K and S, (c) Y and O, (d) Mg and N.
8.18 Which ionic compound is expected to form from combining the following pairs of elements: (a) barium and fluorine, (b) cesium and chlorine, (c) lithium and nitrogen, (d) aluminum and oxygen?
______
8.19 Write the electron configuration for each of the following ions, and determine which ones possess noble-gas configurations: (a) Sr2+, (b) Ti2+, (c) Se2−, (d) Ni2+, (e) Br−, (f) Mn3+.
8.20 Write electron configurations for the following ions, and determine which have noble-gas configurations: (a) Cd2+, (b) P3−, (c) Zr4+, (d) Ru3+, (e) As3−, (f) Ag+.
______
8.21(a) Define the term lattice energy. (b) Which factors govern the magnitude of the lattice energy of an ionic compound?
8.22 NaCl and KF have the same crystal structure. The only difference between the two is the distance that separates cations and anions. (a) The lattice energies of NaCl and KF are given in Table 8.2. Based on the lattice energies, would you expect the Na—Cl or the K—F distance to be longer? (b) Use the ionic radii given in Figure 7.7 to estimate the Na—Cl and K—F distances. Does this estimate agree with the prediction you made based on the lattice energies?
______
8.23 The ionic substances KF, CaO, and ScN are isoelectronic (they have the same number of electrons). Examine the lattice energies for these substances in Table 8.2, and account for the trends you observe.
8.24 (a) Does the lattice energy of an ionic solid increase or decrease (i) as the charges of the ions increase, (ii) as the sizes of the ions increase? (b) Arrange the following substances not listed in Table 8.2 according to their expected lattice energies, listing them from lowest lattice energy to the highest: MgS, KI, GaN, LiBr.
______
8.25 The lattice energies of KBr and CsCl are nearly equal (Table 8.2). What can you conclude from this observation?
8.26 Explain the following trends in lattice energy:
(a) NaCl > RbBr > CsBr; (b) BaO > KF; (c) SrO > SrCl2.
______
8.27 Energy is required to remove two electrons from Ca to form Ca2+ and is required to add two electrons to O to form O2−. Why, then, is CaO stable relative to the free elements?
8.28 List the individual steps used in constructing a Born–Haber cycle for the formation of BaI2 from the elements. Which of the steps would you expect to be exothermic?
______
8.29 Use data from Appendix C, Figure 7.9, and Figure 7.11 to calculate the lattice energy of RbCl. Is this value greater than or less than the lattice energy of NaCl? Explain.
8.30 (a) Based on the lattice energies of MgCl2 and SrCl2 given in Table 8.2, what is the range of values that you would expect for the lattice energy of CaCl2? (b) Using data from Appendix C, Figure 7.9, and Figure 7.11 and the value of the second ionization energy for Ca, 1145 kJ/mol, calculate the lattice energy of CaCl2.
COVALENT BONDING, ELECTRONEGATIVITY, AND BOND POLARITY (sections 8.3 and 8.4)
8.31(a) What is meant by the term covalent bond? (b) Give three examples of covalent bonding. (c) A substance XY, formed from two different elements, boils at −33 °C. Is XY likely to be a covalent or an ionic substance? Explain.
8.32 Which of these elements are unlikely to form covalent bonds: S, H, K, Ar, Si? Explain your choices.
______
8.33 Using Lewis symbols and Lewis structures, diagram the formation of SiCl4 from Si and Cl atoms.
8.34 Use Lewis symbols and Lewis structures to diagram the formation of PF3 from P and F atoms.
______
8.35(a) Construct a Lewis structure for O2 in which each atom achieves an octet of electrons. (b) Explain why it is necessary to form a double bond in the Lewis structure. (c) The bond in O2 is shorter than the O—O bond in compounds that contain an O—O single bond. Explain this observation.
8.36 (a) Construct a Lewis structure for hydrogen peroxide, H2O2, in which each atom achieves an octet of electrons. (b) Do you expect the O—O bond in H2O2 to be longer or shorter than the O—O bond in O2?
______
8.37(a) What is meant by the term electronegativity? (b) On the Pauling scale what is the range of electronegativity values for the elements? (c) Which element has the greatest electronegativity? (d) Which element has the smallest electronegativity?
8.38 (a) What is the trend in electronegativity going from left to right in a row of the periodic table? (b) How do electronegativity values generally vary going down a column in the periodic table? (c) How do periodic trends in electronegativity relate to those for ionization energy and electron affinity?
______
8.39 Using only the periodic table as your guide, select the most electronegative atom in each of the following sets: (a) Na, Mg, K, Ca; (b) P, S, As, Se ; (c) Be, B, C, Si; (d) Zn, Ge, Ga, As.
8.40 By referring only to the periodic table, select (a) the most electronegative element in group 6A; (b) the least electronegative element in the group Al, Si, P; (c) the most electronegative element in the group Ga, P, Cl, Na; (d) the element in the group K, C, Zn, F that is most likely to form an ionic compound with Ba.
______
8.41 Which of the following bonds are polar: (a) B—F, (b) Cl—Cl, (c) Se—O, (d) H—I? Which is the more electronegative atom in each polar bond?
8.42 Arrange the bonds in each of the following sets in order of increasing polarity: (a) C—F, O—F, Be—F; (b) —Cl, S—Br, C—P; (c) C—S, B—F, N—O.
______
8.43(a) From the data in Table 8.3, calculate the effective charges on the H and Br atoms of the HBr molecule in units of the electronic charge, e. (b) Compare your answers to part (a) with those in Sample Exercise 8.5 for the HCl molecule. Can you explain why the values are different?
8.44 The iodine monobromide molecule, IBr, has a bond length of 2.49 Å and a dipole moment of 1.21 D. (a) Which atom of the molecule is expected to have a negative charge? Explain. (b) Calculate the effective charges on the I and Br atoms in IBr, in units of the electronic charge, e.
______
8.45 In the following pairs of binary compounds determine which one is a molecular substance and which one is an ionic substance. Use the appropriate naming convention (for ionic or molecular substances) to assign a name to each compound: (a) SiF4 and LaF3, (b) FeCl2 and ReCl6,(c) PbCl4 and RbCl.
8.46 In the following pairs of binary compounds determine which one is a molecular substance and which one is an ionic substance. Use the appropriate naming convention (for ionic or molecular substances) to assign a name to each compound: (a) TiCl4 and CaF2, (b) ClF3 and VF3, (c)SbCl5 and AlF3.
LEWIS STRUCTURES; RESONANCE STRUCTURES (sections 8.5 and 8.6)
8.47 Draw Lewis structures for the following: (a) SiH4, (b) CO, (c) SF2, (d) H2SO4 (H is bonded to O), , (f) NH2OH.
8.48 Write Lewis structures for the following: (a) H2CO (both H atoms are bonded to C), (b) H2O2, (c) C2F6 (contains a C—C bond), , (e) H2SO3 (H is bonded to O), (f) C2H2.
______

8.49(a) When talking about atoms in a Lewis structure, what is meant by the term formal charge? (b) Does the formal charge of an atom represent the actual charge on that atom? Explain. (c) How does the formal charge of an atom in a Lewis structure differ from the oxidation number of the atom?
8.50 (a) Write a Lewis structure for the phosphorus trifluoride molecule, PF3. Is the octet rule satisfied for all the atoms in your structure? (b) Determine the oxidation numbers of the P and F atoms. (c) Determine the formal charges of the P and F atoms. (d) Is the oxidation number for the P atom the same as its formal charge? Explain.
______
8.51 Write Lewis structures that obey the octet rule for each of the following, and assign oxidation numbers and formal charges to each atom: (a) OCS, (b) SOCl2 (S is bonded to the two Cl atoms and to the O), , (d) HClO2 (H is bonded to O).
8.52 For each of the following molecules or ions of sulfur and oxygen, write a single Lewis structure that obeys the octet rule, and calculate the oxidation numbers and formal charges on all the atoms: (a) SO2, (b) SO3, . (d) Arrange these molecules/ions in order of increasing S—O bond distance.
______
8.53(a) Write one or more appropriate Lewis structures for the nitrite ion, . (b) With what allotrope of oxygen is it isoelec-tronic? (c) What would you predict for the lengths of the bonds in relative to N—O single bonds and double bonds?
8.54 Consider the formate ion, , which is the anion formed when formic acid loses an H+ ion. The H and the two O atoms are bonded to the central C atom. (a) Write one or more appropriate Lewis structures for this ion. (b) Are resonance structures needed to describe the structure? (c) What would you predict for the C—O bond lengths in the formate ion relative to those in CO2?
______
8.55 Predict the ordering of the C—O bond lengths in CO, CO2, and .

8.56 Based on Lewis structures, predict the ordering of N—O bond lengths in NO+, , and .
______
8.57(a) Use the concept of resonance to explain why all six C—C bonds in benzene are equal in length. (b) Are the C—C bond lengths in benzene shorter than C—C single bonds? Are they shorter than C═C double bonds?
8.58 Mothballs are composed of naphthalene, C10H8, a molecule of which consists of two six-membered rings of carbon fused along an edge, as shown in this incomplete Lewis structure:
(a) Write two complete Lewis structures for naphthalene. (b) The observed C—C bond lengths in the molecule are intermediate between C—C single and C═C double bonds. Explain. (c) Represent the resonance in naphthalene in a way analogous to that used to represent it in benzene.
EXCEPTIONS TO THE OCTET RULE (section 8.7)
8.59(a) State the octet rule. (b) Does the octet rule apply to ionic as well as to covalent compounds? Explain using examples as appropriate.
8.60 Considering the nonmetals, what is the relationship between the group number for an element (carbon, for example, belongs to group 4A; see the periodic table on the inside front cover) and the number of single covalent bonds that element needs to form to conform to the octet rule?
______
8.61 The chlorine oxides, in which a chlorine atom is bonded to one or more oxygen atoms, are important molecules in the chemistry of the atmosphere. Will any of the chlorine oxides obey the octet rule? Why or why not?
8.62 For elements in the third row of the periodic table and beyond, the octet rule is often not obeyed. What factors are usually cited to explain this fact?
______
8.63 Draw the Lewis structures for each of the following ions or molecules. Identify those that do not obey the octet rule, and explain why they do not: , (b) AlH3, , (d) CH2Cl2, (e) SbF5.
8.64 Draw the Lewis structures for each of the following molecules or ions. Which do not obey the octet rule? (a) NO, (b) BF3, , (d) OPBr3 (the P is the central atom), (e) XeF4.
______
8.65 In the vapor phase, BeCl2 exists as a discrete molecule. (a) Draw the Lewis structure of this molecule, using only single bonds. Does this Lewis structure satisfy the octet rule? (b) What other resonance structures are possible that satisfy the octet rule? (c) On the basis of the formal charges, which Lewis structure is expected to be dominant for BeCl2?
8.66 (a) Describe the molecule xenon trioxide, XeO3, using four possible Lewis structures, one each with zero, one, two, or three Xe—O double bonds. (b) Do any of these resonance structures satisfy the octet rule for every atom in the molecule? (c) Do any of the four Lewis structures have multiple resonance structures? If so, how many resonance structures do you find? (d) Which of the Lewis structures in (a) yields the most favorable formal charges for the molecule?
______
8.67 Consider the following statement: “For some molecules and ions, a Lewis structure that satisfies the octet rule does not lead to the lowest formal charges, and a Lewis structure that leads to the lowest formal charges does not satisfy the octet rule.” Illustrate this statement using the hydrogen sulfite ion, HSO3-, as an example (the H atom is bonded to one of the O atoms).
8.68 Some chemists believe that satisfaction of the octet rule should be the top criterion for choosing the dominant Lewis structure of a molecule or ion. Other chemists believe that achieving the best formal charges should be the top criterion. Consider the dihydrogen phosphate ion, H2PO4-, in which the H atoms are bonded to O atoms. (a) What would be the predicted dominant Lewis structure if satisfying the octet rule is the top criterion? (b) What would it be if achieving the best formal charges is the top criterion? (c) Is there another Lewis structure you can draw that satisfies neither of these criteria?
BOND ENTHALPIES (section 8.8)
8.69 Using Table 8.4, estimate ΔH for each of the following gas-phase reactions:
8.70 Using Table 8.4, estimate ΔH for the following gas-phase reactions:
Central Atom Hybridization
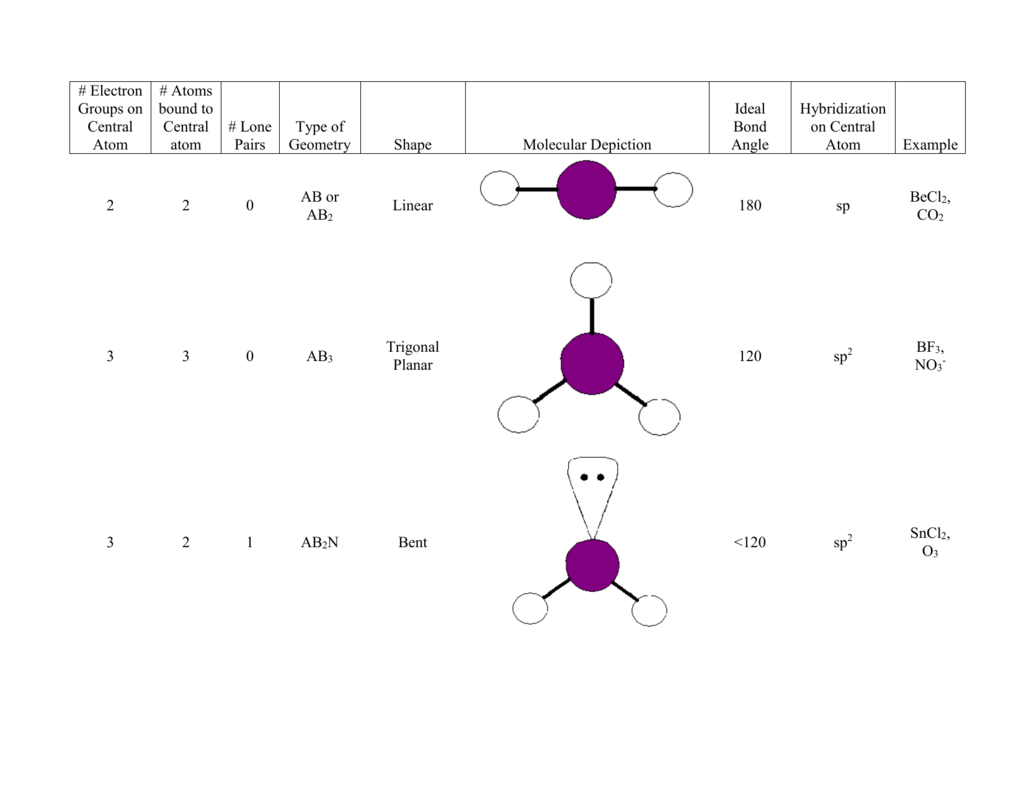
______
8.71 Using Table 8.4, estimate ΔH for each of the following reactions:
(a) 2 CH4(g) + O2(g) → 2 CH3OH(g)
(b) H2(g) + Br2(g) → 2 HBr(g)
(c) 2 H2O2(g) → 2 H2O(g) + O2(g)
8.72 Use Table 8.4 to estimate the enthalpy change for each of the following reactions:
(a) H2C=O(g) + HCl(g) → H3C—O—Cl(g)
(b) H2O2(g) + 2 CO(g) → H2(g) + 2 CO2(g)
(c) 3 H2C═CH2(g) → C6H12(g) (the six carbon atoms form a six-membered ring with two H atoms on each C atom)
______
8.73 Ammonia is produced directly from nitrogen and hydrogen by using the Haber process. The chemical reaction is
N2(g) + 3H2(g) → 2 NH3(g)
(a) Use Table 8.4 to estimate the enthalpy change for the reaction. Is it exothermic or endothermic? (b) Compare the enthalpy change you calculate in (a) to the true enthalpy change as obtained using values.
8.74 (a) Use bond enthalpies to estimate the enthalpy change for the reaction of hydrogen with ethylene:
H2(g) + C2H4(g) → C2H6(g)
(b) Calculate the standard enthalpy change for this reaction, using heats of formation. Why does this value differ from that calculated in (a)?
______
8.75 Given the following bond-dissociation energies, calculate the average bond enthalpy for the Ti—Cl bond.
[8.76] (a) Using average bond enthalpies, predict which of the following reactions will be most exothermic:
(i) C(g) + 2 F2(g) → CF4(g)
(ii) CO(g) + 3 F2 → CF4(g) + OF2(g)
(i) CO2(g) + 4 F2 → CF4(g) + 2OF2(g)
(b) Explain the trend, if any, that exists between reaction exothermicity and the extent to which the carbon atom is bonded to oxygen.
ADDITIONAL EXERCISES
8.77 How many elements in the periodic table are represented by a Lewis symbol with a single dot? Are all these elements in the same group? Explain.

[8.78] From Equation 8.4 and the ionic radii given in Figure 7.7, calculate the potential energy of the following pairs of ions. Assume that the ions are separated by a distance equal to the sum of their ionic radii: (a) Na+, Br-; (b) Rb+, Br-; (c) Sr2+, S2−.
8.79 (a) Explain the following trend in lattice energy: BeH2, 3205 kJ/mol; MgH2, 2791 kJ/mol; CaH2, 2410 kJ/mol; SrH2, 2250 kJ/mol; BaH2, 2121 kJ/mol. (b) The lattice energy of ZnH2 is 2870 kJ/mol. Based on the data given in part (a), the radius of the Zn2+ ion is expected to be closest to that of which group 2A element?
8.80 Based on data in Table 8.2, estimate (within 30 kJ/mol) the lattice energy for (a) LiBr, (b) CsBr, (c) CaCl2.
8.81 An ionic substance of formula MX has a lattice energy of 6 × 103 kJ/mol. Is the charge on the ion M likely to be 1+, 2+ or 3+? Explain your reasoning.
[8.82] From the ionic radii given in Figure 7.7, calculate the potential energy of a Ca2+ and O2− ion pair that is just touching (the magnitude of the electronic charge is given on the back inside cover). Calculate the energy of a mole of such pairs. How does this value compare with the lattice energy of CaO (Table 8.2)? Explain the difference.
8.83 Construct a Born–Haber cycle for the formation of the hypothetical compound NaCl2, where the sodium ion has a 2+ charge (the second ionization energy for sodium is given in Table 7.2). (a) How large would the lattice energy need to be for the formation of NaCl2 to be exothermic? (b) If we were to estimate the lattice energy of NaCl2 to be roughly equal to that of MgCl2 (2326 kJ/mol from Table 8.2), what value would you obtain for the standard enthalpy of formation, , of NaCl2?
8.84 (a) How does a polar molecule differ from a nonpolar one? (b) Atoms X and Y have different electronegativities. Will the diatomic molecule X—Y necessarily be polar? Explain. (c) What factors affect the size of the dipole moment of a diatomic molecule?
8.85 For the following collection of nonmetallic elements, O, P, Te, I, B, (a) which two would form the most polar single bond? (b) Which two would form the longest single bond? (c) Which two would be likely to form a compound of formula XY2? (d) Which combinations of elements would likely yield a compound of empirical formula X2Y3? In each case explain your answer.
8.86 The substance chlorine monoxide, ClO(g), is important in atmospheric processes that lead to depletion of the ozone layer. The ClO molecule has a dipole moment of 1.24 D and the Cl—O bond length is 1.60 Å. (a) Determine the magnitude of the charges on the Cl and O atoms in units of the electronic charge, e. (b) Based on the electronegativities of the elements, which atom would you expect to have a negative charge in the ClO molecule? (c) By using formal charges as a guide, propose the dominant Lewis structure for the molecule. Are the formal charges consistent with your answers to parts (a) and (b)? Can you reconcile any differences you find?
[8.87] Using the electronegativities of Br and Cl, estimate the partial charges on the atoms in the Br—Cl molecule. Using these partial charges and the atomic radii given in Figure 7.7, estimate the dipole moment of the molecule. The measured dipole moment is 0.57 D.
8.88 A major challenge in implementing the “hydrogen economy” is finding a safe, lightweight, and compact way of storing hydrogen for use as a fuel. The hydrides of light metals are attractive for hydrogen storage because they can store a high weight percentage of hydrogen in a small volume. For example, NaAlH4 can release 5.6% of its mass as H2 upon decomposing to NaH(s), Al(s), and H2(g). NaAlH4 possesses both covalent bonds, which hold polyatomic anions together, and ionic bonds. (a) Write a balanced equation for the decomposition of NaAlH4. (b) Which element in NaAlH4 is the most electronegative? Which one is the least electronegative? (c) Based on electronegativity differences, what do you think is the identity of the polyatomic anion? Draw a Lewis structure for this ion.
8.89 Although is known, is not. Using Lewis structures, explain why F3− does not form.
8.90 Calculate the formal charge on the indicated atom in each of the following molecules or ions: (a) the central oxygen atom in O3, (b) phosphorus in , (c) nitrogen in NO2, (d) iodine in ICl3, (e) chlorine in HClO4 (hydrogen is bonded to O).
8.91 (a) Determine the formal charge on the chlorine atom in the hypochlorite ion, ClO-, and the perchlorate ion, , using resonance structures where the Cl atom has an octet. (b) What are the oxidation numbers of chlorine in ClO- and in ? (c) Is it uncommon for the formal charge and the oxidation state to be different? Explain. (d) Perchlorate is a much stronger oxidizing agent than hypochlorite. Would you expect there to be any relationship between the oxidizing power of the oxyanion and either the oxidation state or the formal charge of chlorine?
8.92 The following three Lewis structures can be drawn for N2O:
(a) Using formal charges, which of these three resonance forms is likely to be the most important? (b) The N—N bond length in N2O is 1.12 Å, slightly longer than a typical N≡N bond; and the N—O bond length is 1.19 Å, slightly shorter than a typical N=O bond. (See Table 8.5.) Rationalize these observations in terms of the resonance structures shown previously and your conclusion for part (a).
[8.93] (a) Triazine, C3H3N3, is like benzene except that in triazine every other C—H group is replaced by a nitrogen atom. Draw the Lewis structure(s) for the triazine molecule. (b) Estimate the carbon-nitrogen bond distances in the ring.
[8.94] Ortho-dichlorobenzene, C6H4Cl2, is obtained when two of the adjacent hydrogen atoms in benzene are replaced with Cl atoms. A skeleton of the molecule is shown here. (a) Complete a Lewis structure for the molecule using bonds and electron pairs as needed. (b) Are there any resonance structures for the molecule? If so, sketch them. (c) Are the resonance structures in (a) and (b) equivalent to one another as they are in benzene? If not, explain what makes them different.
Electron Groups Around Central Atom
8.95 Consider the hypothetical molecule B—A=B. How could you use an experimentally determined structure of the molecule to decide whether resonance is important in it?
8.96 An important reaction for the conversion of natural gas to other useful hydrocarbons is the conversion of methane to ethane.
In practice, this reaction is carried out in the presence of oxygen, which converts the hydrogen produced to water.
Use Table 8.4 to estimate ΔH for these two reactions. Why is the conversion of methane to ethane more favorable when oxygen is used?
8.97 Two compounds are isomers if they have the same chemical formula but a different arrangement of atoms. Use Table 8.4 to estimate ΔH for each of the following gas-phase isomerization reactions, and indicate which isomer has the lower enthalpy:
[8.98] With reference to the “Chemistry Put to Work” box on explosives, (a) use bond enthalpies to estimate the enthalpy change for the explosion of 1.00 g of nitroglycerin. (b) Write a balanced equation for the decomposition of TNT. Assume that, upon explosion, TNT decomposes into N2(g), CO2(g), H2O(g), and C(s).
[8.99] The “plastic” explosive C-4, often used in action movies, contains the molecule cyclotrimethylenetrinitramine, which is often called RDX (for Royal Demolition eXplosive):
Cyclotrimethylenetrinitramine (RDX)
(a) Complete the Lewis structure for the molecule by adding unshared electron pairs where they are needed. (b) Does the Lewis structure you drew in part (a) have any resonance structures? If so, how many? (c) The molecule causes an explosion by decomposing into CO(g), N2(g), and H2O(g). Write a balanced equation for the decomposition reaction. (d) With reference to Table 8.4, which is the weakest type of bond in the molecule? (e) Use average bond enthalpies to estimate the enthalpy change when 5.0 g of RDX decomposes.
8.100 The bond lengths of carbon-carbon, carbon-nitrogen, carbon-oxygen, and nitrogen-nitrogen single, double, and triple bonds are listed in Table 8.5. Plot bond enthalpy (Table 8.4) versus bond length for these bonds (as in Figure 8.17). What do you conclude about the relationship between bond length and bond enthalpy? What do you conclude about the relative strengths of C—C, C—N, C—O, and N—N bonds?
INTEGRATIVE EXERCISES
8.101 The Ti2+ ion is isoelectronic with the Ca atom. (a) Are there any differences in the electron configurations of Ti2+ and Ca? (b) With reference to Figure 6.24, comment on the changes in the ordering of the 4s and 3d subshells in Ca and Ti2+. (c) Will Ca and Ti2+ have the same number of unpaired electrons? Explain.
[8.102] (a) Write the chemical equations that are used in calculating the lattice energy of SrCl2(s) via a Born-Haber cycle. (b) The second ionization energy of Sr(g) is 1064 kJ/mol. Use this fact along with data in Appendix C, Figure 7.9, Figure 7.11, and Table 8.2 to calculate ΔH°f for SrCl2(s).
[8.103] The electron affinity of oxygen is –141 kJ/mol, corresponding to the reaction
The lattice energy of K2O(s) is 2238 kJ/mol. Use these data along with data in Appendix C and Figure 7.9 to calculate the “second electron affinity” of oxygen, corresponding to the reaction
8.104 You and a partner are asked to complete a lab entitled “Oxides of Ruthenium” that is scheduled to extend over two lab periods. The first lab, which is to be completed by your partner, is devoted to carrying out compositional analysis. In the second lab, you are to determine melting points. Upon going to lab you find two unlabeled vials, one containing a soft yellow substance and the other a black powder. You also find the following notes in your partner's notebook—Compound 1: 76.0% Ru and 24.0% O (by mass), Compound 2: 61.2% Ru and38.8% O (by mass). (a) What is the empirical formula for Compound 1? (b) What is the empirical formula for Compound 2? (c) Upon determining the melting points of these two compounds, you find that the yellow compound melts at 25 °C, while the black powder does not melt up to the maximum temperature of your apparatus, 1200 °C. What is the identity of the yellow compound? What is the identity of the black compound? Be sure to use the appropriate naming convention depending on whether the compound is better described as a molecular or ionic compound.
[8.105] One scale for electronegativity is based on the concept that the electronegativity of any atom is proportional to the ionization energy of the atom minus its electron affinity: electronegativity = k(IE − EA), where k is a proportionality constant. (a) How does this definition explain why the electronegativity of F is greater than that of Cl even though Cl has the greater electron affinity? (b) Why are both ionization energy and electron affinity relevant to the notion of electronegativity? (c) By using data in Chapter 7, determine the value of k that would lead to an electronegativity of 4.0 for F under this definition. (d) Use your result from part (c) to determine the electronegativities of Cl and O using this scale. Do these values follow the trend shown in Figure 8.7?
8.106 The compound chloral hydrate, known in detective stories as knockout drops, is composed of 14.52% C, 1.83% H, 64.30% Cl, and 19.35% O by mass and has a molar mass of 165.4 g/mol. (a) What is the empirical formula of this substance? (b) What is the molecular formula of this substance? (c) Draw the Lewis structure of the molecule, assuming that the Cl atoms bond to a single C atom and that there are a C—C bond and two C—O bonds in the compound.
8.107 Barium azide is 62.04% Ba and 37.96% N. Each azide ion has a net charge of 1−. (a) Determine the chemical formula of the azide ion. (b) Write three resonance structures for the azide ion. (c) Which structure is most important? (d) Predict the bond lengths in the ion.
8.108 Acetylene (C2H2) and nitrogen (N2) both contain a triple bond, but they differ greatly in their chemical properties. (a) Write the Lewis structures for the two substances. (b) By referring to Appendix C, look up the enthalpies of formation of acetylene and nitrogen and compare their reactivities. (c) Write balanced chemical equations for the complete oxidation of N2 to form N2O5(g) and of acetylene to form CO2(g) and H2O(g). (d) Calculate the enthalpy of oxidation per mole of N2 and C2H2 (the enthalpy of formation of N2O5(g) is 11.30 kJ/mol). How do these comparative values relate to your response to part (b)? Both N2 and C2H2 possess triple bonds with quite high bond enthalpies (Table 8.4). What aspect of chemical bonding in these molecules or in the oxidation products seems to account for the difference in chemical reactivities?
[8.109] Under special conditions, sulfur reacts with anhydrous liquid ammonia to form a binary compound of sulfur and nitrogen. The compound is found to consist of 69.6% S and 30.4% N. Measurements of its molecular mass yield a value of 184.3 g mol−1. The compound occasionally detonates on being struck or when heated rapidly. The sulfur and nitrogen atoms of the molecule are joined in a ring. All the bonds in the ring are of the same length. (a) Calculate the empirical and molecular formulas for the substance. (b) Write Lewis structures for the molecule, based on the information you are given. (Hint: You should find a relatively small number of dominant Lewis structures.) (c) Predict the bond distances between the atoms in the ring. (Note: The S—S distance in the S8 ring is 2.05 Å.) (d) The enthalpy of formation of the compound is estimated to be 480 kJ mol−1. of S(g) is 222.8 kJ mol−1. Estimate the average bond enthalpy in the compound.
[8.110] A common form of elemental phosphorus is the tetrahedral P4 molecule, where all four phosphorus atoms are equivalent:
At room temperature phosphorus is a solid. (a) Do you think there are any unshared pairs of electrons in the P4 molecule? (b) How many P—P bonds are there in the molecule? (c) Can you draw a Lewis structure for a linear P4 molecule that satisfies the octet rule? (d) Using formal charges, what can you say about the stability of the linear molecule versus that of the tetrahedral molecule?
[8.111] Consider benzene (C6H6) in the gas phase. (a) Write the reaction for breaking all the bonds in C6H6(g), and use data in Appendix C to determine the enthalpy change for this reaction. (b) Write a reaction that corresponds to breaking all the carbon-carbon bonds in C6H6(g). (c)By combining your answers to parts (a) and (b) and using the average bond enthalpy for C H from Table 8.4, calculate the average bond enthalpy for the carbon-carbon bonds in C6H6(g). (d) Comment on your answer from part (c) as compared to the values for C—C single bonds and C═C double bonds in Table 8.4.
8.112 Average bond enthalpies are generally defined for gas-phase molecules. Many substances are liquids in their standard state. (Section 5.7) By using appropriate thermochemical data from Appendix C, calculate average bond enthalpies in the liquid state for the following bonds, and compare these values to the gas-phase values given in Table 8.4: (a) Br—Br, from Br2(l); (b) C—Cl, from CCl4(l); (c) O—O, from H2O2(l) (assume that the O—H bond enthalpy is the same as in the gas phase). (d) What can you conclude about the process of breaking bonds in the liquid as compared to the gas phase? Explain the difference in the ΔH values between the two phases.
Molecular Structures
Lewis Structure, Molecular Formula, Ball-and-Stick model, Space-filling Model, Structural Formula
A structural formula is a molecular model that uses letter symbols and bonds to show relative positions of atoms. It can generally be predicted by means of a Lewis structure. The following steps can be used in drawing Lewis structures.
Apply these steps in each case.(Using the compound CF4 For our example)
STEP 1
Add up all of the valences electrons from each atom in the compound.Find the total number of electrons available for bonding (valence electrons). If the structure is to represent a positive or negative polyatomic ion, the ion charge must be subtracted or added, respectively.
CF4 = 4 + 4(7) = 32 electrons
STEP 2
Divide the total number of available electrons by 2 to obtain the number of bonding pairs.
Example: 32 electrons ÷ 2 = 16 pairs
Central Atom Of Nh3
* Why do we do this? Well, remember back when we learned orbital notations. There was always two electrons per orbital because of opposite spin…
STEP 3
Next, we designate the central atom. The other atoms become terminal atoms and should be placed around the central atom. Predict the location of certain atoms. Hydrogen is always a terminal atom. The atom with the least attraction for electrons is the central atom (Least electronegative).
The atom that is the least electronegative is the one central atoms (unless it’s H, because H can only be a terminal atom.)
STEP 4
Place one bonding pair between the central atom and each terminal atom. Connect the terminal atoms with a “ –”. This represents a shared pair of electrons, so subtract that number from your previous total.
16 pairs - 4 shared pairs = 12 pairs
A shared pair comes from the atoms overlapping their electron clouds in order to complete their individual Octets.
STEP 5
Now, those remaining electron pairs need to be assigned. Begin placing them around the terminal atoms until you complete the Octet. To find the total number of lone pairs and pairs available for multiple bonding, subtract the number of bonding pairs used in step 4 from the number of bonding pairs determined in step 2. Place lone pairs around the terminal atoms to satisfy the octet rule. Assign remaining pairs to the central atom.
12 pairs - 12 lone pairs = 0 remaining pairs
These dots are called lone pairs or more commonly, nonbonding pairs. These can vastly alter the geometry when they go on the central atom.
STEP 6
If there are any electron pairs left over after step 5, put them around the central atom.
Example: XeF4
CAUTION: This is called having an “expanded Octet”. The central atoms that can do this are found on row three and below (B, C, N, O, F, Ne cannot do this!)
What exception to the octet rule is this example?
KrCl6
Expanded octet
STEP 7
Finally, before you’re done, make sure the central atom has a complete Octet. If the central atom is not surrounded by four electron pairs, convert one or two lone pairs on the terminal atoms to a double or triple bond to the central atom. If it does not, and you are out of lone pairs to add…you must take a lone pair from a terminal atom and make a double bond.
Example: SiO2
Example : Drawing Lewis Structures
Draw Lewis structures for each of the following.
a. phosphorus trichloride (PCl3)
b. sulfate ion (SO42-)
Practice
Central Atom Co2
9. Draw Lewis structures for each of the following.
a. CF4
b. CO
c. SiS2
d. NH4+
Answers on unit objectives page.
Draw Lewis structures
Resonance and exceptions to the octet rule
Resonance occurs when more than one valid Lewis structure can be written for a molecule or an ion. For example, three resonance structures exist for the NO3- ion because a double bond can be placed between the central N atom and any of the three O atoms. Must have multiple bonds and lone pairs that appear to “swap” positions . Bonds, molecules don’t resonate! Molecules exhibit resonance. Most significant (“best”) resonance structures minimize formal charge, places negative charge on more electronegative atom.
Sometimes there are exceptions to the octet rule. There may be an odd number of total valence electrons, as in the case of ClO2.
A central atom may have more or fewer than eight electrons.
Practice
10. Draw the three resonance structures for the carbonate ion (CO32-). (Hint: Each structure contains one double bond.)
11. State why each of the following is an exception to the octet rule.
a. NO2
b. BCl3
c. PF5
Answers on unit objectives page.
Formal Charge - Bookkeeping method to account for valence electrons.
Formal charge = electrical charge difference between the number of valence electrons in an isolated neutralatom and the number of electrons assigned to it in a Lewis structure.
- Different from oxidation number!
- All non-bonding (lone pair) electrons on an atom are assigned to it
- Half of all bonding electrons (= # of bonds) are assigned to it
- Formal Charge = # Valence electrons – (# bonds + # non-bonding electrons)
The formal charges in a Lewis Structure must add up to the overall charge on the structure.
Interpret Lewis Structures
Determine formal charges
